For the second time within the historical past of HIV infection, a patient appears to have been cured fully of the infection which eventually causes AIDS.
Twelves years in the past, to the day, news concerning the first patient cured of HIV was announced — something researchers had been struggling to do for a really very long time. After a number of trials, failures and repeat experiments, researchers have lastly had success with the second case of medically-treated remission from HIV an infection.
The researcher group is due to publish their findings in Nature soon, and provides a short presentation about their work in the Convention on Retroviruses and Opportunistic Infections in Seattle.
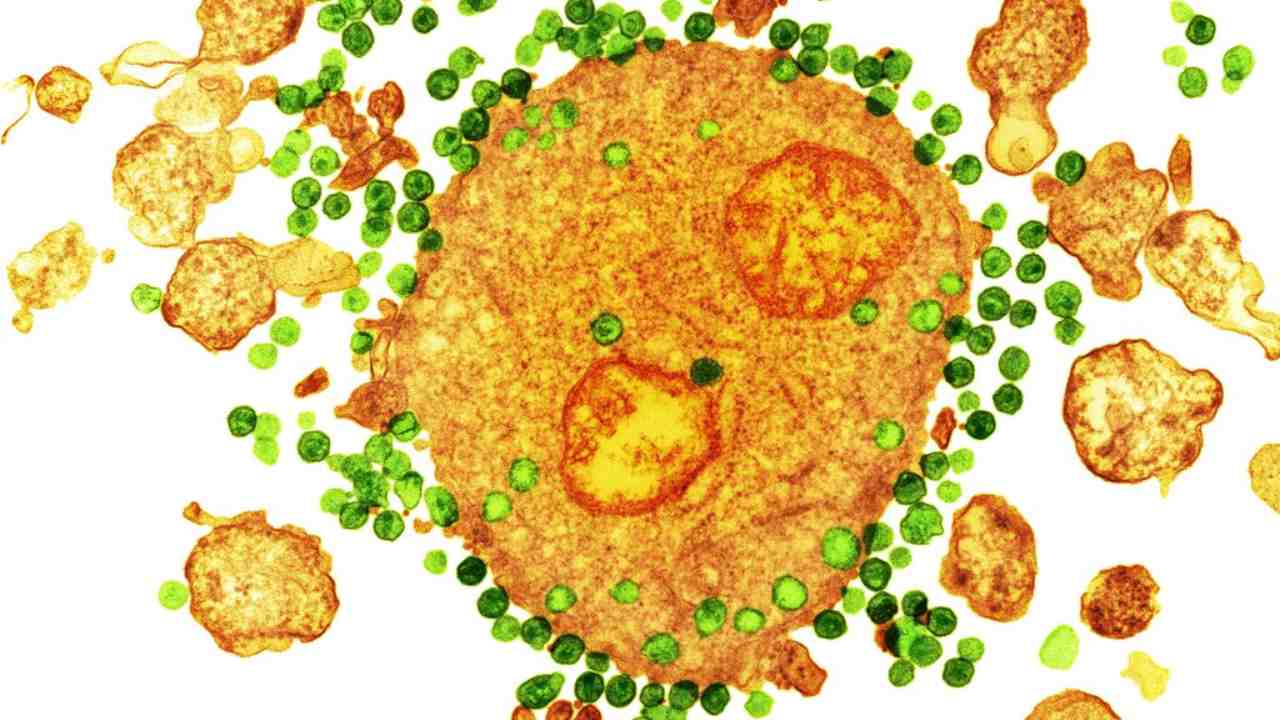 |
| The HIV virus, in green, attaching to a white blood cell, in orange, as seen under a coloured transmission electron microscope. Image credit: NIBSC[/caption] |
Specialists and researchers speaking about this second case, often called the "London Patient", are publicly stating that the patient is "in long-term remission", not "cured". The caveat to the terms is that there have solely ever been two instances of the phenomenon throughout medical history, so that they're merely not sure what the appropriate terminology is as yet.
In both instances, the HIV-infected patient was treated utilizing bone-marrow transplants that have been really designed to treat cancer patients and not HIV. This makes it a tough and unfeasible possibility as a therapy for other HIV-positive patients within the near future.
Bone-marrow transplants could have harsh side-effects that last years, not to point out a much larger risk of developing cancer. At present, there are highly effective and efficient medication obtainable to control HIV infection with few or no side-effects.
"This will inspire people that cure is just not a dream," Dr Annemarie Wensing, a virologist at the University Medical Center Utrecht in the Netherlands, told the New York Times. "It’s reachable."After news of the first "Berlin patient" broke on the same Seattle Conference in 2007, scientists have been trying hard to replicate the results in other HIV-infected patients. In each case till now, either the virus roared again to life — often around 9 months after getting off their treatment, or the patients died of cancer.
The Berlin patient was recognized many years later as Timothy Ray Brown, a 52-year-old man now a California resident. In Brown's case, he had leukaemia and was in need of bone-marrow transplants since his chemotherapy was failing him. Fortunately for him, his donor had a mutation in a gene known as CCR5, which is linked with some individuals having a natural resistance to HIV. CCR5 can also be the gene that Chinese researcher He Jiankui tweaked in embryos to provide them a genetically-engineered resistance to HIV infection throughout their lifetimes.
With immunosuppressive medication and repeated bone marrow transplants, the therapy ultimately worked. However Brown nearly died by the end of the treatment.
 |
| Timothy Brown, the first man to have once had HIV and then not, thanks to medicine. Image courtesy: Center for Health Journalism |
"We’ve always puzzled whether all that conditioning... an enormous quantity of destruction to his immune system... explained why Timothy was cured but nobody else."Now, the London patient is proof {that a} near-death experience is not essentially a part of the process. His transplant beat cancer without any threatening side-effects, and the transplanted immune cells that have been made immune to HIV appeared to have changed all of the HIV-vulnerable cells in his blood.
While there is not one hundred percent assure, the London patient has been off his medication for a year without any sign of the virus making a comeback. She/he/ze appears to have adopted the identical restoration path as Mr Brown all those years in the past after their treatments.
Mr Brown hopes that the London patient’s cure proves as durable as his own, NYT reported. "If something has occurred once in medical science, it could possibly happen again," he stated. "I’ve been waiting for company for a very long time."





















